Offshore Cathodic Protection 101:
What it is and how it works
Richard Baxter and Jim Britton
How does steel corrode in water?
To understand cathodic protection, you must first understand how corrosion is caused. For corrosion to occur, three things must be present:
1. Two dissimilar metals
2. An electrolyte (water with any type of salt or salts dissolved in it)
3. A metal (conducting) path between the dissimilar metals
The two dissimilar metals may be totally different alloys – such as steel and aluminum – but are more likely to be microscopic or macroscopic metallurgical differences on the surface of a single piece of steel. In this case we will consider freely-corroding steel, which is non-uniform.
If the above conditions exist, the following reaction takes place at the more active sites: (two iron ions plus four free electrons).
2Fe => 2Fe++ + 4e-
The free electrons travel through the metal path to the less active sites, where the following reaction takes place: (oxygen gas is converted to oxygen ion - by combining with the four free electrons - which combines with water to form hydroxyl ions).
O2 + 4e- + 2H20 => 4 OH-
Recombinations of these ions at the active surface produce the following reaction, which yields the iron-corrosion product ferrous hydroxide: (iron combining with oxygen and water to form ferrous hydroxide).
2Fe + O2 + 2H2O => 2Fe (OH)2
This reaction is more commonly described as 'current flow through the water from the anode (more active site) to the cathode (less active site).'
How does cathodic protection stop corrosion?
Cathodic protection prevents corrosion by converting all of the anodic (active) sites on the metal surface to cathodic (passive) sites by supplying electrical current (or free electrons) from an alternate source.
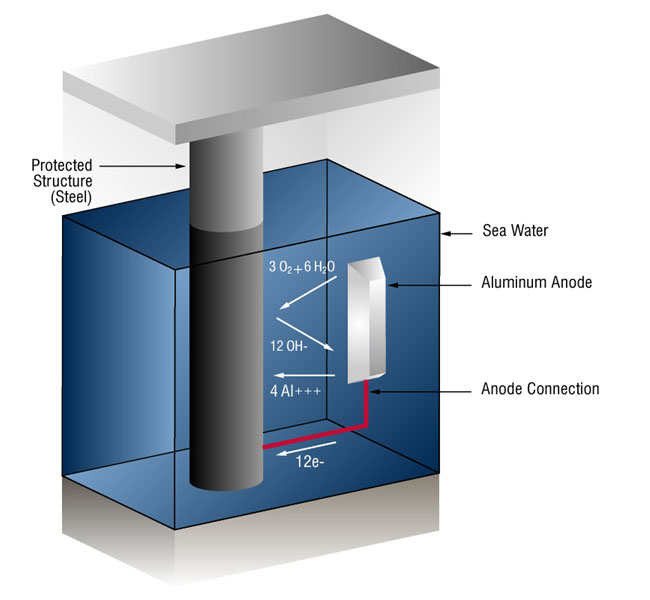
Usually this takes the form of galvanic anodes, which are more active than steel. This practice is also referred to as a sacrificial system, since the galvanic anodes sacrifice themselves to protect the structural steel or pipeline from corrosion.
In the case of aluminum anodes, the reaction at the aluminum surface is: (four aluminum ions plus twelve free electrons)
4Al => 4AL+++ + 12 e-
and at the steel surface: (oxygen gas converted to oxygen ions which combine with water to form hydroxyl ions).
3O2 + 12e- + 6H20 => 12OH-
As long as the current (free electrons) arrives at the cathode (steel) faster than oxygen is arriving, no corrosion will occur.
Basic considerations when designing sacrificial anode systems
The electrical current an anode discharges is controlled by Ohm's law, which is:
I=E/R
I= Current flow in amps
E= Difference in potential between the anode and cathode in volts
R= Total circuit resistance in ohms
Initially, current will be high because the difference in potential between the anode and cathode are high, but as the potential difference decreases due to the effect of the current flow onto the cathode, the current gradually decreases due to polarization of the cathode. The circuit resistance includes both the water path and the metal path, which includes any cable in the circuit. The dominant value here is the resistance of the anode to the seawater.
For most applications, the metal resistance is so small compared to the water resistance that it can be ignored (although this is not true for sleds or long pipelines protected from both ends). In general, long, thin anodes have lower resistance than short, fat anodes. They will discharge more current but will not last as long.
Therefore, a cathodic-protection designer must size the anodes so that they have the right shape and surface area to discharge enough current to protect the structure and enough weight to last the desired lifetime when discharging this current.
As a general rule of thumb:
The length of the anode determines how much current the anode can produce, and consequently, how many square feet of steel can be protected. The cross section (weight) determines how long the anode can sustain this level of protection.
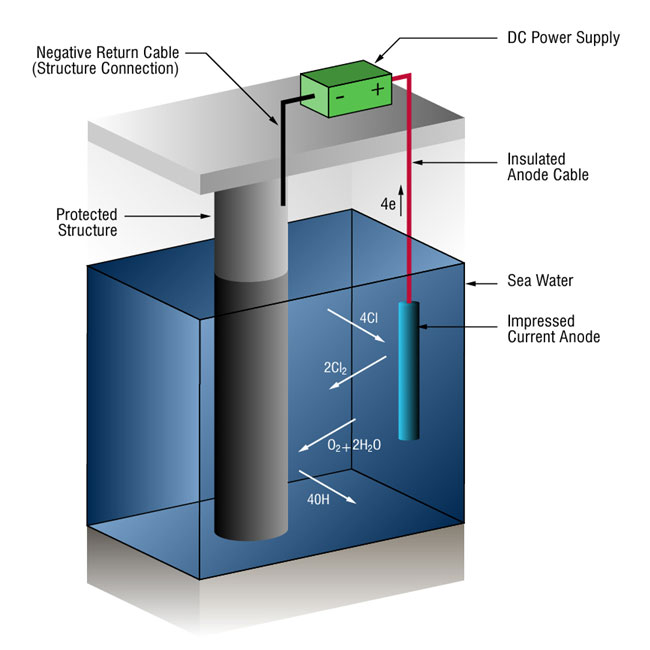
Impressed-current cathodic protection systems (ICCP anode systems)
Due to the high currents involved in many seawater systems, it is not uncommon to use impressed-current systems that use anodes of a type (ICCP anodes) that are not easily dissolved into metallic ions. This causes an alternative reaction: the oxidization of the dissolved chloride ions.
2Cl- => Cl2 + 2e-
Power is supplied by an external DC power unit.
How do we know when we have enough cathodic protection?
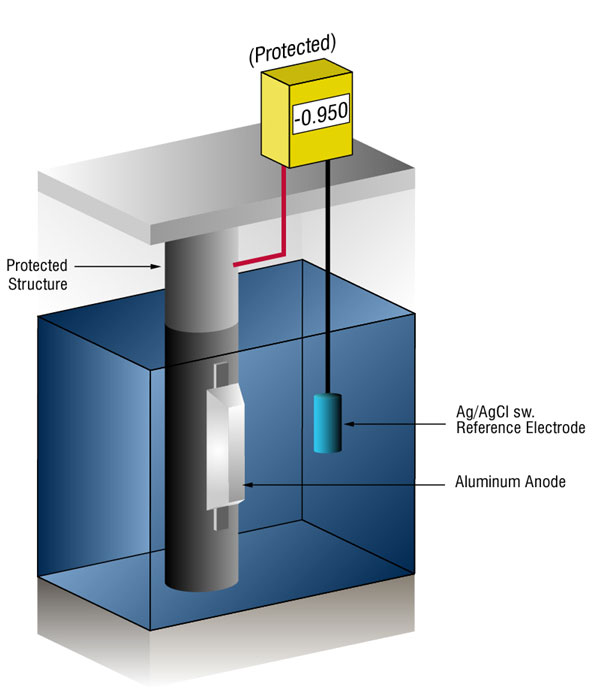
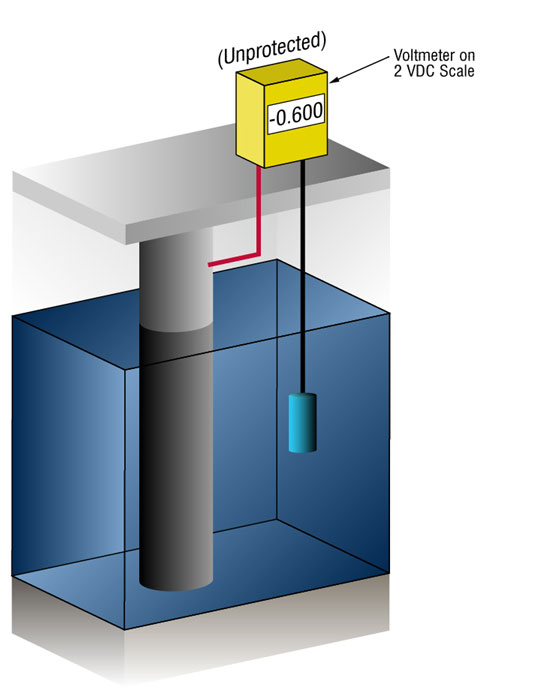
We can verify that there's enough current by measuring the potential of the steel against a standard reference electrode, usually silver silver/chloride (Ag/AgCl sw.), but sometimes zinc (sw.).
Current flow onto any metal will shift its normal potential in the negative direction. History has shown that if steel receives enough current to shift the potential to (-) 0.800 V vs. silver / silver chloride (Ag / AgCl), the corrosion is essentially stopped.
Due to the nature of the films which form, the minimum (-0.800 V) potential is rarely optimum, so designers try to achieve a potential between (-) 0.950 V and (-) 1.000 V vs. Ag/AgCl sw.
 |
 |









